Molecular Pathology
Introduction
Molecular pathology is a critical component of modern healthcare. Analyzing tissues and cells at the histological, DNA, RNA, and proteomic levels produces important diagnostic and prognostic information that can improve health outcomes.
In parallel with clinical diagnostics in the hospital, the study of animal model and patient tissue samples in the laboratory is essential to the biomedical research enterprise. Tissue-derived data serve as a gold standard against which to confirm or refute predictions from simple model systems (e.g., isolated molecules or cultured cell lines), and as a template for primary discoveries of molecular function that are only evident in ex vivo specimens or in vivo systems.
The Institute works in the molecular pathology field as a major long-term theme, which enables appreciation of the arc of biological discovery, technology invention, commercialization, clinical use, overcoming silos, and sunsetting of methods. For both clinical diagnostics and biomedical research, the ethos of Avoneaux Med is, “the more molecular pathology tools the better,” since there is not a one-size-fits-all technological approach sufficient for the many cell types, organelles, and pathologies that exist.
Key questions we address are:
- How to design technologies that permit sophisticated analysis of tissue samples to extend and complement studies of cultured cell lines and purified biomolecules performed in the laboratory?
- How to overcome the clinical-laboratory divide to facilitate the study of tissue specimens by basic scientists?
- How to address the spatial complexity of the tissue microenvironment to gain new knowledge about disease development and progression?
- How to address problems around effective anti-cancer drug delivery to tumors?
Avoneaux Med Technologies
Projects that the Institute is/was involved with include the following areas. For readers interested in additional information or technical/methodological details, linked references are provided below.
Laser Capture Microdissection (LCM) – A technology for procuring normal or diseased cells from histology sections for subsequent molecular study.
- Immuno-LCM
- Computer-Aided Laser Dissection (e.g., Spatially Invariant Vector Quantitation)
Expression Microdissection – A method for retrieving cells from histology slides that is related to LCM but uses molecular targeting in place of human-based cell selection.
Micropurification – A minimalist technique for procuring cells from histology sections using molecular targeting and solution-based processing.
Layered Expression Scanning; 2D-PCR – Two technologies for measuring the molecular content of histology slides while preserving spatial information.
Dynamic Magnetic Shift – A drug delivery strategy addressing the problem of inadequate tissue levels of anti-cancer drugs in the tumor microenvironment.
Molecular Pathology Proteomics – A strategy to measure and analyze the proteins in tissue that are responsible for normal cell functioning and pathophysiology.
Laser Capture Microdissection - Cell Procurement
Goal
Create a technology that changes the cell procurement paradigm from crude slide scraping and other manual tools to precise, laser-based target cell recovery.
History
Laser Capture Microdissection (LCM) was invented in the 1990s in the NIH intramural program in Bethesda, MD, and a manuscript describing the technology was published in the journal Science in 1996. Key inventors and contributors at NIH included Drs. Lance Liotta, Robert Bonner, Zhengping Zhuang, Rhonda Weiss, Paul Smith, Seth Goldstein, and Mr. Thomas Pohida. A public-private partnership was formed with Dr. Thomas Baer who used the NIH patent covering LCM and venture capital funding from Silicon Valley to launch a new company, Arcturus Engineering, Inc.
The first commercial LCM instrument became available on the market in 1997 and the field rapidly expanded, including production of instruments from both startup companies and well established corporations (see Figure below). The NIH inventors of LCM worked closely with Arcturus Engineering for several years, a synergistic arrangement that produced multiple new LCM advancements along with associated commercial reagents useful for analyzing small numbers of dissected cells.
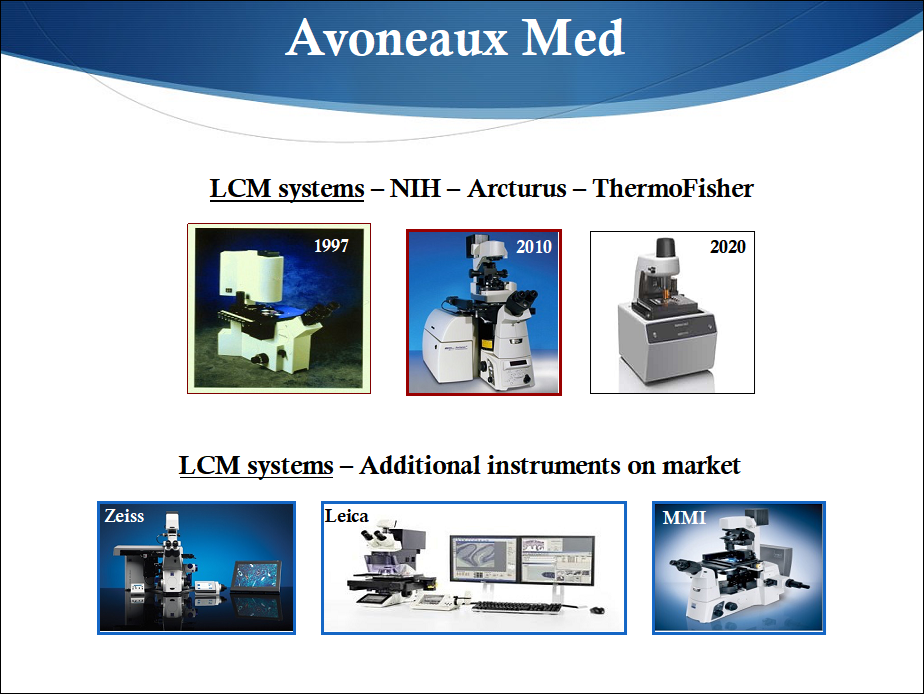
Since the late 1990s to today, LCM and accessory methods from Arcturus Engineering and the many other good companies producing LCM instruments resulted in over 5,000 basic science publications, produced significant sales revenue, and employed a large number of people in the US and Europe. As a specific example, the Institute and collaborators, notably Dr. G. Steven Bova, studied prostate cancer using LCM to generate novel insights that were not evident using alternative strategies. (For additional details, see the references below).
New Approaches
Despite the advancements over the past 20 years, there are challenges that remain for the field, including the time, effort, and cost associated with LCM, which can make using the technology impractical for some applications. Importantly, a pathologist or trained histology expert is frequently required to identify target cells in histology slides, which can hinder use of the method by laboratory scientists such as biochemists or molecular biologists.
Thus, Avoneaux Med and a large cadre of other investigators/companies in the US and worldwide continue to develop new dissection tools and associated methods for research and clinical applications. These include improved protocols for LCM, along with immuno-based and other automated technologies and instrumentation. Ultimately, the goal is to create new cell procurement methods that are efficient, inexpensive, and do not require histology expertise. Ideally, such methods could be performed by clinicians and scientists in their own laboratories and on their own benchtops to maximally ‘democratize’ procurement capability.
For example, immuno-LCM combines immunostained tissue with LCM and allow investigators to target specific cell types by visually aiming a laser at immunohistochemical (IHC)- or immunofluorescence (IF)-stained cells. The method proved useful for numerous studies, including epigenetic analysis, tumor microenvironment studies, and neuroscience research.
Alternatively, spatially invariant vector quantitation-LCM (SIVQ-LCM) is but one example of a computer-aided laser dissection (CALD) technique that allows one to select phenotypic features (size, architecture, texture, color) of target cells and then search for similar cells across a histological section. Together with Drs. Jason Hipp and Ulysses Balis at the University of Michigan, and Jeffrey Hanson and Jaime Rodriguez-Canales at the NIH, SIVQ was adapted to the Arcturus XT instrument to create a new auto-dissection tool that reduces time and effort demands by shifting investigators to a supervisory role rather than a 'shot-by-shot' laser operator.
Expression Microdissection
This technology represents a conceptual advance for dissection of cells using molecular targeting that was invented and developed in the NIH intramural program in Bethesda, MD in collaboration with Drs. Michael Tangrea and Robert Bonner, and builds upon LCM instrumentation and methods. However, unlike LCM, no human- or computer-based visualization of cells is required, thus the efficiency is increased by using label-based targeting.
Micropurification
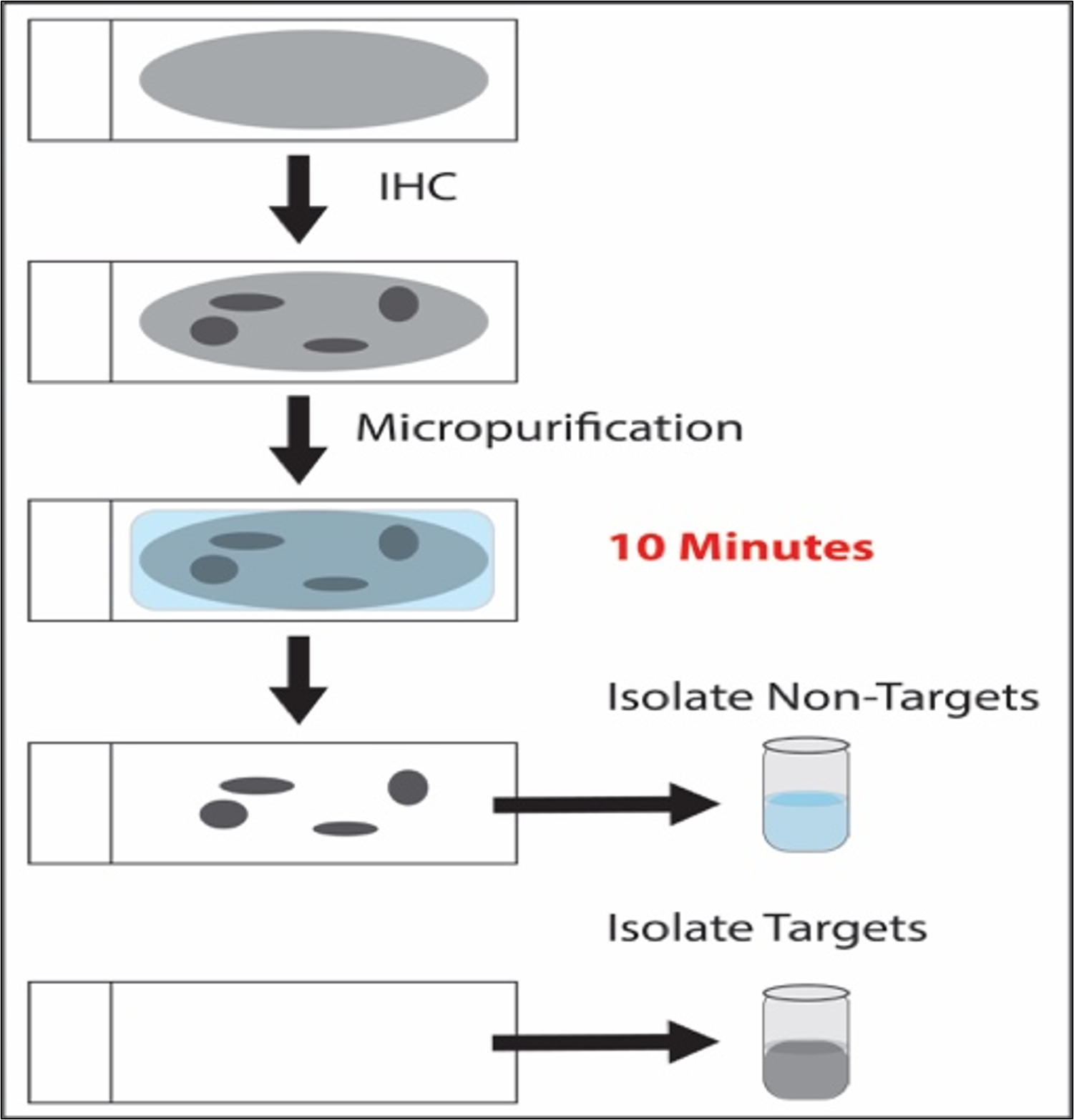
The most recent dissection method developed, micropurification is especially simple and minimalist. The technology recovers target cells or subcellular structures from a histological section based on molecular characteristics and without the need for instrumentation or expert-based slide annotation. Micropurification was invented and developed at Avoneaux Med with Drs. Michael Tangrea, Sarah Laun, and David Krizman, and with Marlene Darfler, Kathleen Bengali, James Salvatierra, Devin Demelis, and Anitha Chalasani. A schematic drawing outlining the process is shown in the Figure below. The upper panel is a non-labeled histological section on a glass slide. All the cells and extracellular structures are light gray in color. Unique molecular characteristics of target cells allow targeting by a probe (e.g., an antibody) followed by deposition of an insoluble barrier on the target cells (darkly colored). A micropurification solution is then applied to the slide to remove all non-protected cells, which can be collected and analyzed. The target cells remain on the slide and are subsequently placed into a separate tube for molecular analysis. Importantly, all target cells, structures, or subcellular organelles are collected during the procedure, allowing rapid procurement of large numbers of cell components for either analytic or preparative applications.
Selected References - Cell Procurement
- Laser Capture Microdissection. Science. 1996 Nov 8;274(5289):998-1001. doi: 10.1126/science.274.5289.998. PMID: 8875945.
- Laser Capture Microdissection: Molecular analysis of tissue. Science. 1997 Nov 21;278(5342):1481,1483. doi: 10.1126/science.278.5342.1481. PMID: 9411767.
- The genetics of cancer--a 3D model. Nat Genet. 1999 Jan;21(1 Suppl):38-41. doi: 10.1038/4466. PMID: 9915499.
- Post-analysis follow-up and validation of microarray experiments. Nat Genet. 2002 Dec;32 Suppl:509-14. doi: 10.1038/ng1034. PMID: 12454646.
- Quantitative RT-PCR gene expression analysis of laser microdissected tissue samples. Nat Protoc. 2009;4(6):902-22. doi: 10.1038/nprot.2009.61. Epub 2009 May 21. PMID: 19478806; PMCID: PMC2760821.
- Expression microdissection adapted to commercial laser dissection instruments. Nat Protoc. 2011 Apr;6(4):457-67. doi: 10.1038/nprot.2010.202. Epub 2011 Mar 18. PMID: 21412274; PMCID: PMC4795005.
- Analysis of transcription factor mRNAs in identified oxytocin and vasopressin magnocellular neurons isolated by laser capture microdissection. PLoS One. 2013 Jul 24;8(7):e69407. doi: 10.1371/journal.pone.0069407. PMID: 23894472; PMCID: PMC3722287.
- The evolutionary history of lethal metastatic prostate cancer. Nature. 2015 Apr 16;520(7547):353-357. doi: 10.1038/nature14347. Epub 2015 Apr 1. Erratum in: Nature. 2020 Aug;584(7820):E18. doi: 10.1038/s41586-020-2581-5. PMID: 25830880; PMCID: PMC4413032.
- Similar image search for histopathology: SMILY. NPJ Digit Med. 2019 Jun 21;2:56. doi: 10.1038/s41746-019-0131-z. PMID: 31304402; PMCID: PMC6588631.
- Prostate cancer evolution from multilineage primary to single lineage metastases with implications for liquid biopsy. Nat Commun. 2020 Oct 8;11(1):5070. doi: 10.1038/s41467-020-18843-5. PMID: 33033260; PMCID: PMC7545111.
- Expression Microdissection: Operator-independent retrieval of cells for molecular profiling. Diagn Mol Pathol. 2004 Dec;13(4):207-12. doi: 10.1097/01.pdm.0000135964.31459.bb. PMID: 15538110.
- High-throughput microdissection for next-generation sequencing. PLoS One. 2016 Mar 21;11(3):e0151775. doi: 10.1371/journal.pone.0151775. PMID: 26999048; PMCID: PMC4801357.
- Computer-aided laser dissection: A microdissection workflow leveraging image analysis tools. J Pathol Inform. 2018 Dec 11;9:45. doi: 10.4103/jpi.jpi_60_18. PMID: 30622835; PMCID: PMC6298131.
- Micropurification – A new method to isolate cell targets from histological sections. Meeting Poster.
American Society of Investigative Pathology (ASIP), Baltimore, MD, April 2024.
Layered Expression Scanning (LES); 2D-PCR - Spatial Analysis
Standard LCM, immuno-LCM, CALD, xMD, and micropurification enable examination of specific cell populations in histological sections. However, by whatever means performed, dissection methods are not completely adequate for studying tissue as important spatial information can be lost by pooling dissected cells together prior to analysis. Consider for example a field of invasive tumor. In the future it may become clinically important to identify the worst sub-component of a tumor or tumor microenvironment based on a molecular signature, as opposed to determining an aggregate profile from all tumor cells. Since one does not know a priori if or where unique tumor sub-populations or reactive host cells exist, there is a need for techniques that permit the spatial molecular content of whole tissue sections to be surveyed and analyzed.
LES was developed to address the challenge of integrating histopathology with spatial multiplex expression measurements, combining tissue sections with a third-dimension molecular array. The method works by transferring an intact histological section through a layered set of membranes, each of which measures an individual transcript or protein. LES was invented at NIH, reduced-to-practice by Dr. Chad Englert and Ms. Galina Baibokov, and licensed by NIH to help start a new company, 20/20 Gene Systems, Inc.
A second method to facilitate spatial tissue analysis is 2D-PCR, which was invented in the NIH Intramural Program in Bethesda and co-developed by Drs. Michael Armani and Michael Tangrea at NIH, along with Drs. Benjamin Shapiro and Elisabeth Smela at the University of Maryland at College Park. The goal is to improve the nucleic acid amplification and measurement process while preserving important two-dimensional cellular relationships. The method transfers an intact tissue section into a high-density, multi-well PCR grid that provides an optimized aqueous environment for nucleic acid manipulation. After thermocycling is complete the amplified products are measured and the data integrated with histological features of the tissue sample. The method can be preformed as a stand alone procedure, and/or integrated with micropurification technology to permit sub-regional cell procurement, i.e., 'spatial micropurification.'
Selected LES and 2D-PCR References
- Layered expression scanning: rapid molecular profiling of tumor samples. Cancer Res. 2000 Mar 15;60(6):1526-30. PMID: 10749117.
- Layered expression scanning: multiplex analysis of RNA and protein gels. Biotechniques. 2003 Dec;35(6):1280-5. doi: 10.2144/03356pf01. PMID: 14682064.
- Layered expression scanning: multiplex molecular analysis of diverse life science platforms. Clin Chim Acta. 2007 Feb;376(1-2):9-16. doi: 10.1016/j.cca.2006.08.007. Epub 2006 Aug 11. PMID: 16996046.
- 2D-PCR: a method of mapping DNA in tissue sections. Lab Chip. 2009 Dec 21;9(24):3526-34. doi: 10.1039/b910807f. Epub 2009 Oct 12. PMID: 20024032; PMCID: PMC2910845.
- Quantifying mRNA levels across tissue sections with 2D-RT-qPCR. Anal Bioanal Chem. 2011 Jul;400(10):3383-93. doi: 10.1007/s00216-011-5062-8. Epub 2011 May 11. PMID: 21559756; PMCID: PMC7375691.
Dynamic Magnetic Shift – Anti-Cancer Drug Delivery
The three-dimensional tumor microenvironment is a barrier to drug delivery due to the rapid and uncontrolled growth of cancer cells, which produces a disorganized and only partially functional biological milieu. One outcome of this process is an abnormal vascular system. Unlike the well-structured series of small vessels that create a fine meshwork of capillaries in normal tissues to deliver oxygen and nutrients within a diffusion-limited distance of cells, tumors often exhibit a complex and disordered blood supply, resulting in diminished perfusion to some or all parts of the tumor microenvironment and reduced delivery of systemically administered therapeutic agents.
In collaboration with Drs. Ben Shapiro and Alek Nacev at the University of Maryland at College Park, a method was designed to achieve two important goals: (1) increase drug levels in poorly vascularized tumors or tumor subregions by equalizing the concentration between tumor and normal tissues, and (2) improve drug levels simultaneously in all tumor foci across a given anatomical region, without the need for imaging-based, positional information of lesions.
To evaluate feasibility, histological liver sections from autopsy cases of women who died from breast neoplasms were studied to measure vessel number, size, and spatial distribution in both metastatic tumors and normal tissue. Numerical simulations of magnetically driven particle transport based on the autopsy data were performed and indicated DMS can significantly increase nanoparticle levels in hypovascular regions of metastases.
The Figure below is a schematic illustration of DMS showing increased nanoparticle levels into and throughout liver metastatic tumor foci. Left and right panels: Magnets create gradients on therapeutic nanoparticles to displace them from well perfused normal tissue (a continually refilling source of drug) into adjacent poorly vascularized tumor regions. In this example, magnetic shift is shown in just two successive directions, but the process can be repeated in multiple spatial planes. Middle panels: Computer simulations of the therapeutic particle distributions in a 1 mm wide tissue region using the blood vessel (small dark structures) geometry taken from autopsy data. The color gradient shows the resulting nanoparticle concentration at each tissue location (red is high, yellow is medium, white is low). Magnetic actuation increases nanoparticle concentration in the tumor area (marked by the black circle, also clearly visible by a lack of blood vessels) at 30, 60, 120, and 180 minutes after systemic injection (from top left middle panel to bottom right).
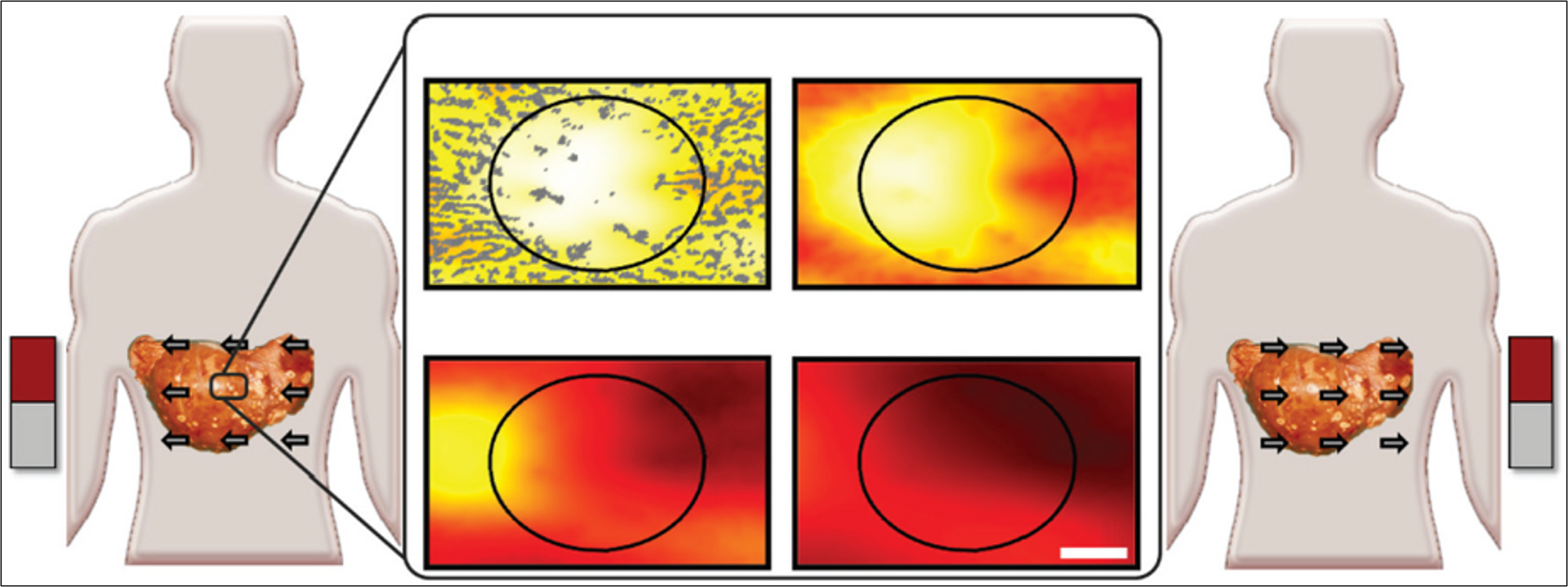
Overall, DMS may have utility for improving delivery of therapeutics to primary tumors and disseminated metastases in a tumor number/location agnostic fashion. The method is a promising strategy for further development.
Selected DMS References
- A dynamic magnetic shift method to increase nanoparticle concentration in cancer metastases: a feasibility study using simulations on autopsy specimens. Int J Nanomedicine. 2011;6:2907-23. doi: 10.2147/IJN.S23724. Epub 2011 Nov 18. PMID: 22131836; PMCID: PMC3224717.
- Towards Control of Magnetic Fluids in Patients: Directing Therapeutic Nanoparticles to Disease Locations, June 2012, IEEE Control Systems Magazine. 32(3):32, DOI: 10.1109/MCS.2012.2189052
Molecular Pathology Proteomics
The study of proteins in clinical tissue samples is uniquely useful in understanding pathophysiology since proteins are the key biomolecules that carry out most downstream cellular functions.
Expression Pathology, Inc was formed along with Dr. Michael Amos, Dr. David Krizman, and Ms. Marlene Darfler to advance proteomic measurements of dissected cells and structures in clinical tissue specimens. The company invented several novel approaches to facilitate the process and was later renamed Nantomics (https://nantomics.com) after acquisition by the Nant Health System. The company now functions as mProbe (https://www.mprobe.com) in Rockville, MD, and offers companion diagnostic proteomic assays to guide patient care.
